The Tipping Point: Issue 7
by Jess Spear
The Tipping Point is a regular column on recent climate and biodiversity news.
Wildfires in our oceans
“You have heatwave-induced wildfires that take out huge areas of forest, but this is happening underwater as well.”[1]
As regular Rupture readers know, I write a lot about the ocean.[2] This is partly because I’ve always been captivated by this big blue space, out there, inhabited by all kinds of weird and amazing life. The first Blue Planet documentary series was released when I was in college, and I can vividly remember sitting on my dorm room bed taping all eight episodes, pausing to skip commercials, just so I could rewatch them later. (This was way before everything was available on demand of course!) In graduate school, I studied marine science, taught oceanography to primary school students and mentored young teenagers at an oceanography camp for girls. So, yeah, I really love the ocean. But I don’t write about the ocean because I personally find it fascinating. I write about it because I want Rupture readers to understand that there is no safe future for us without a healthy ocean.
Approximately ninety per cent of all heat trapped by rising greenhouse gases has gone into the ocean. This has led to sea level rise[3], supercharged hurricanes and increased rainfall. For marine life, though, the effects have been devastating. You could probably withstand a day or two of 40-degree weather, but month after month? Year after year? Probably not. Most people know about coral bleaching, whereby prolonged exposure to extreme heat compels corals to expel the algae living in their tissues[4]. This not only removes their colour but also their ability to feed themselves. How many of you have heard about the mysterious mass sea star deaths from “sea star wasting syndrome”?[5]
A new study[6] released earlier this year reveals just how much heat has accumulated over the last 150 years, and the results are extremely worrying.
““By 2014, the global ocean was at a “point of no return” whereby 50% of the global sea surface was hotter than the baseline.””
The scientists involved wanted to understand how extreme heat affects marine ecosystems and how much of the ocean is affected. To do this, they looked up the hottest monthly temperatures recorded in the top layer of the ocean (that is, sea surface temperature, SST) from 1870-1919. They found that extreme heat occurred just two days out of every 100 during that period. This is then used as a baseline from which they can assess how hot the ocean has become since. They also calculated how many days and how much of the region was continually hotter than the baseline.
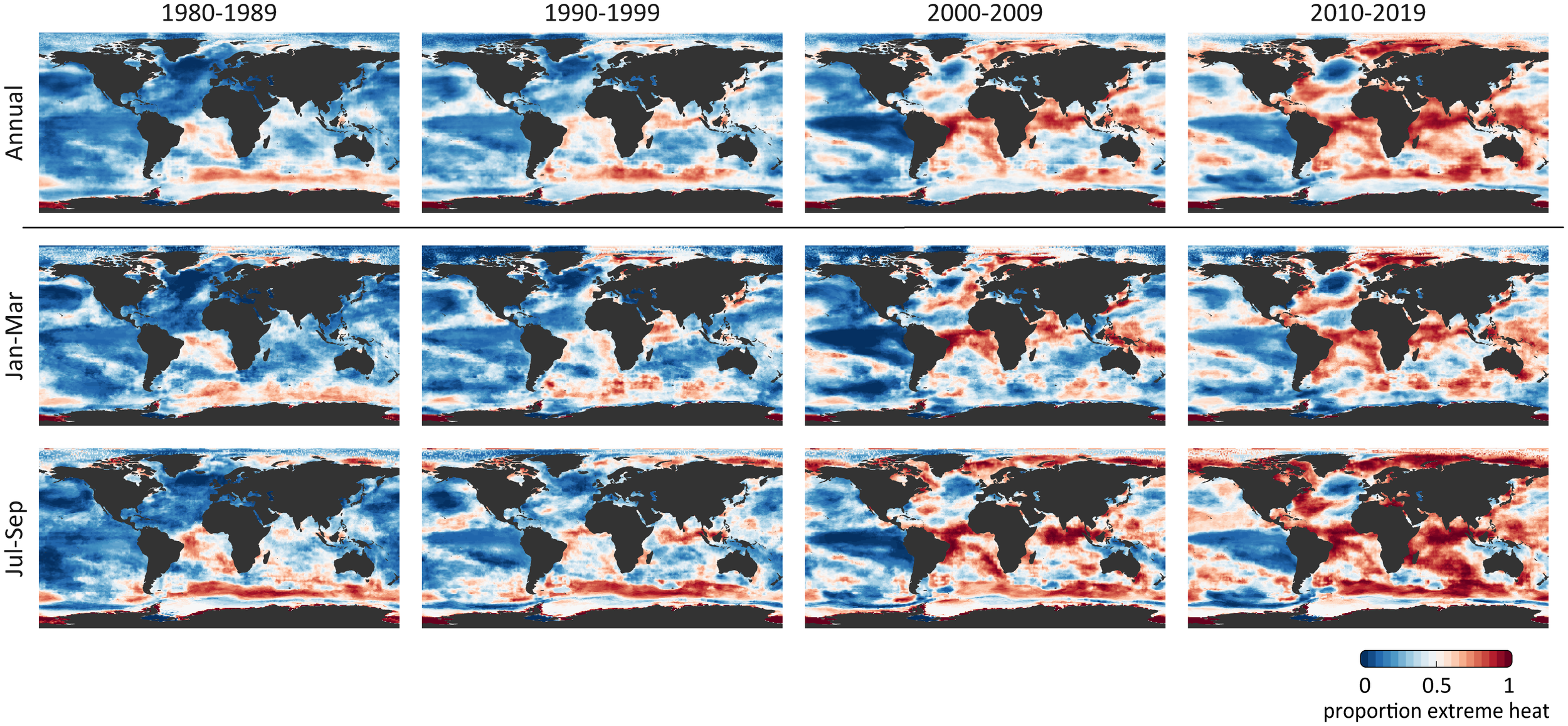
You can see in the figure below that from 1980 to 2019 more and more of the ocean is experiencing extreme heat, particularly the Indian, Arctic, and Southern Oceans. They discovered that by 2014, the global ocean was at a “point of no return” whereby 50% of the global sea surface was hotter than the baseline (that is, the hottest temperatures between 1870-1919).
Figure 1. How extreme heat has developed in the global ocean over the last 40 years. The top row shows the average annual temperatures; the second row is winter temperatures (Jan, Feb, Mar); and the third row is summer temperatures (Jul, Aug, Sep). The more red the area, the higher the temperature relative to the 1870-1919 baseline.
Crossing the chemical pollution threshold
"Better Things for Better Living...Through Chemistry." DuPont Chemicals slogan from 1935-1982
I grew up in the late 80s and early 90s. All the girls my age wore their hair in this kind of wave that required copious amounts of the strongest hairspray you could find. Every morning before school, my older (and much cooler) sister would show me how to get the perfect wave. First, you’d hike up your fringe with a comb, hairspray the hell out of it, and then shape it until it curved perfectly across your forehead. Mine never looked as good as hers.
On one such occasion, my mom’s friend watched as we emptied a bottle of Aquanet, desperate to get the lift and bend just right before the hair-hardened rock solid. “You know that destroys the ozone, right?” she said.
I was only 10. I didn’t know what an “ozone” was or why we shouldn’t destroy it. I just wanted my hair to look like Kelly Kapowski’s on Saved by the Bell.
““I just wanted my hair to look like Kelly Kapowski’s on ‘Saved by the Bell’.” ”
Little did I know that, just a few years earlier, a young scientist with the British Antarctic Survey discovered that the ozone layer above Antarctica had lost approximately ⅓ of its thickness. This was after scientists had warned that the chemical in my Aquanet hairspray, a chlorofluorocarbon otherwise known as CFCs, could destroy ozone. This chemical was originally manufactured by DuPont scientist and "one-man environmental disaster"[7] Thomas Midgely Jr., who also invented leaded gasoline.
Like many manufactured chemicals, CFCs were thought to be relatively harmless because they were “chemically inert” which means they don’t readily react with the surrounding environment. To demonstrate this, Midgely Jr. actually “inhaled a large amount of the gas, and then blew out a candle flame.” Originally, CFCs were created to replace much more dangerous chemicals used to keep refrigerators cold, but then they were added to all kinds of products, hairspray, deodorants, and even inhalers. The problem is that CFCs found their way to a very different environment than your average bathroom, the upper atmosphere. There, they were no longer inert. They readily reacted with ozone, ripping apart molecule after molecule.
As if this wasn’t bad enough, CFCs are also a very potent greenhouse gas. Their heat-trapping potential is 11,000 times greater than CO2; and, since their lifespan in the upper atmosphere is 55-100 years, we are still dealing with the effects decades after the global phaseout.
““They readily reacted with ozone, ripping apart molecule after molecule.””
And that’s the crux of it. Chemical companies manufacture new compounds that become widespread, making the company millions, if not billions in profits. It's only after years or even decades, and after many tons of it are released into the environment, that we learn of its harmful effects. Plastics take centuries to break down and yet companies continue to pump them out like there’s no tomorrow.
That brings me to a recent study that quantified the planetary boundary for chemical pollution or “novel entities”. Since 2009 scientists have been investigating what is the “safe operating space for humanity”. In other words, how much change could be sustained before the environment was fundamentally altered and no longer safe for humanity? Ten boundaries have been identified: climate change, biodiversity loss, nitrogen cycle, phosphorus, ocean acidification, land use, freshwater, ozone depletion, atmospheric aerosols, and chemical pollution. Until now, scientists had yet to quantify the chemical pollution boundary. Well, now they have; and, spoiler alert, we passed it.
Taking the most comprehensive survey possible, researchers found that “annual production and releases are increasing at a pace that outstrips the global capacity for assessment and monitoring”. This is because “[p]roduction of novel entities…increased 50-fold since 1950, and is projected to triple again by 2050 compared to 2010. Furthermore, “[t]here are an estimated 350,000 chemicals (or mixtures of chemicals) on the global market. Nearly 70,000 have been registered in the past decade.”[8]
We are literally surrounded by manufactured chemicals and “novel entities”. One of the researchers on the study, Prof Bethanie Carney Almroth points out that “the total mass of plastics now exceeds the total mass of all living mammals. That to me is a pretty clear indication that we’ve crossed a boundary.”
Extractivism in the ocean
“We have never entered a frontier and not fucked it up more.”[9]
We used to think the deep ocean had no life. It’s understandable, really. We can’t survive down there. It’s too cold and there’s too much pressure. Also, sunlight can’t penetrate deeper than about 200 metres, so how could any animals survive if the plant life they rely on can’t photosynthesize? In 1843 Edward Forbes, a naturalist aboard the HMS Beacon, postulated that since he found fewer and smaller species the deeper he sampled, no life existed below 550 metres.
His theory was disproved not long after when other scientists dredged greater depths and found life. Still, it wasn’t until the HMS Challenger voyage in the 1870s that we discovered just how deep the ocean was and how many species inhabited even the deepest regions.[10] Modern oceanography is only about 70 years old and, in the US in particular, has largely been driven by military needs (mapping the seafloor, underwater acoustics).[11]
Very few people have ever visited the deepest region, in the Mariana Trench. (Though, for the hefty price tag of $750,000, the super-rich can now become ocean explorers.)[12] To this day, scientists continue to find new species and new ecosystems.
““Modern oceanography is only about 70 years old and, in the US in particular, has largely been driven by military needs.””
New species of octopus discovered in 2016 living at 4,290 metres (14,075 feet). Credit: NOAA National Marine Fisheries Service
For example, in the 1970s, researchers discovered a diverse array of life grouped around hydrothermal vents - areas where seawater seeps through cracks in the seafloor created by seafloor spreading or where the seafloor is plunging beneath another plate. They’re kind of like underwater volcanoes. Once the water comes into contact with magma it’s superheated and bursts out full of heavy metals, some of which rapidly cool to form chimney-like structures, but also are used by microbes to create energy in a process called chemosynthesis. These microbes provide the basis for an entire ecosystem built around hydrothermal vents which include giant tubeworms, shrimp, and limpets.
So, there is still so much to learn about life in the deep sea and its connection to life above. But that’s not stopping a tiny island in the middle of the Pacific, Nauru, already environmentally degraded from decades of phosphate mining, from teaming up with a Canadian mining company to begin ploughing the seafloor.
Once the country alerted the International Seabed Authority (ISA) of its intention to start mining, the ISA has just two years to finalise regulations before mining can literally just begin. (As of writing, there are only 16 months left.) Nauru and their mining company partners, The Metals Company - super original name, right? - are trying to pressure the ISA to finalise regulations they’ve been discussing since 2014, and were due to finish in 2020 if not for the Covid pandemic. But, that doesn’t mean the ISA is the saviour here, boldly standing up to mining companies eyeing big profits and protecting fragile ecosystems. They’ve never rejected any exploration applications, for example. Clearly, we need a ban on deep-sea mining, not just regulations, rubber stamping projects, and poor oversight.
The company and supporters of deep sea mining argue that we need the minerals to rapidly transition away from fossil fuels. Deep-sea mining is more environmentally friendly, they say, than the extraction of minerals on land. We can do it, they say, in a way that won’t damage the environment. But, “[a]ny claim of not being environmentally damaging is meaningless,” says Will McCalluma of UK Greenpeace oceans division, because “we have no idea now what that environment is”.[13]
Image: GEOMAR
While we have much more to learn, what we already know is enough to demonstrate the stupidity of moving ahead. Firstly, some of the deposits targeted by mining companies are found around the hydrothermal vents I described above. These regions clearly support unique ecosystems. Secondly, at least for Nauru, it is the polymetallic nodules that offer the best prospect for mineral collection. These are “dark, potato-sized mineral masses or concretions, [which] form slowly by the precipitation of metals from seawater and the sediment underneath. They grow several millimetres every million years and sit on the seafloor like ‘cobbles on a street,’ which are lying on seafloors”.[14] While collecting these nodules won’t require drilling per se, that doesn’t mean harvesting them is harmless. The nodules themselves “act like trees in a forest”[15] creating habitat for animals such as sea cucumbers and brittle stars. No forest, no animals, no ecosystem.